The fractional composition of the 12C/13C et 16O/18O isotopes in carbon dioxide (CO2), and in the carbonates from which one can extract, and thus measure, the carbon dioxide, is a very popular method used since may nears in the Earth sciences.
A very recent technique concentrates in particular on the distribution of these isotopes for the four varieties of the same molecule : the most abundant CO2 isotope (12C16O2), the versions including a rare isotope (13C instead of 12C or 18O instead of 16O) and the rare varieties, 13C16O18O, with a double substitution of a heavy isotope.
In thermodynamic equilibrium, the redistribution of these four isotopes within the four varieties depends entirely on the temperature. On the other hand, it does not depend on the other environmental variables (pH, rapports 13C/12C et 18C/16O…), so that this tracer constitutes a particularly robust and interesting thermometer.
Until now, this kind of measurement was assured by specialized mass spectrometry, which enables one to determine the relative abundance of the very rare 13C16O18O to an accuracy of 10-5.
The new instrumental research carried out by the Franco-german team is based on the use of an infra-red laser in order to measure the relative abundances of these four CO2 isotopes.
For the first time, the temperature of the CO2 dissolved in the thermal water of these three different sources in the upper Rhine basin (one at Soultz/Alsace and two others at Bad Hombourg/Hesse) has been measured by this new technique which uses the equilibrium composition of the four isotopes 12C16O2, 13C16O2, 12C16O18O et 13C16O18O.
In the case of two of these three cases, the temperature corresponds to that of surface water, while at Soultz, the temperature is much higher, a consequence of fast convection in the water. The double measurement using mass spectrometry and via laser has led to the conclusion that the laser measurement is almost as accurate for comparable sampling times.
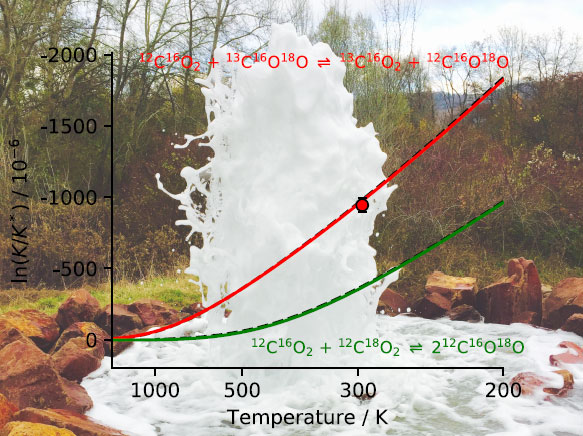
On the basis of this clear and very promising experiment, the scientists believe that an improved instrument will enable one in the near future to reach an accuracy close to that of mass spectrometry, while reducing the measurement time from several hours to about ten minutes. The new method is so promising because the measurement is direct. Comparing just the relative abundances of the four isotopes, one can deduce the equilibrium temperature unambiguously, as shown by a comparison of experiment and theoretical calculation.
The direct measurement is much better than that using mass spectrometry, which needs additional information and calibration. The team believes that their method will even enable one to measure the very rare isotopic varieties such as 12C18O2, which so far has never been detected.
Source(s) :
I. Prokhorov, T. Kluge, C. Janssen, Optical clumped isotope thermometry of carbon dioxide, Scientific Reports (2019) doi : 10.1038/s41598-019-40750-z
